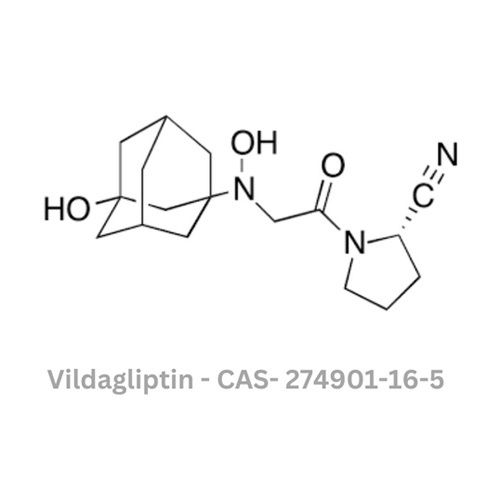
Vildagliptin API
Product Details:
- Structural Formula Available on request
- Solubility Freely soluble in methanol, slightly soluble in water
- Melting Point 209-214C
- Poisonous NO
- Shelf Life 2 years from date of manufacture
- Particle Size D90 < 150 microns
- HS Code 29420090
- Click to View more
X
Vildagliptin API Product Specifications
- Bitter
- Odorless
- 303.40 g/mol
- C17H25N3O2
- Store in a cool, dry place, protected from light
- Pharmaceutical Grade
- Vildagliptin
- 0.001% (10 ppm)
- Active Pharmaceutical Ingredient (API)
- White to off-white
- >= 99%
- 274901-16-5
- (S)-1-[N-(3-Hydroxy-1-adamantyl)glycyl]pyrrolidine-2-carbonitrile
- 29420090
- Solid
- Antidiabetic agent, used in the treatment of type 2 diabetes mellitus
- D90 < 150 microns
- Not applicable (decomposes)
- 0.5%
- White to off-white powder
- 209-214C
- NO
- Freely soluble in methanol, slightly soluble in water
- Available on request
- 2 years from date of manufacture
Vildagliptin API Trade Information
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
Product Description
Get the grandiose Vildagliptin API, a first-rate antidiabetic agent crafted for pharmaceutical brilliance. With breathtaking purity (99%) and tightly controlled impurities (0.2%), this limited time offer ensures exceptional quality for your formulations. Identified by HPLC and IR spectroscopy, Vildagliptin is delivered in solid, white to off-white powder form, odorless and potent. Our GMP-compliant facility ensures each batch meets rigorous standards, and a Certificate of Analysis is provided. Grab yours now in secure HDPE drums and experience unparalleled performance in type 2 diabetes treatment!
Vildagliptin API: Powerful Antidiabetic Excellence
Vildagliptin API is used for the effective management of type 2 diabetes mellitus, delivering pronounced glycemic control in pharmaceutical formulations. Its competitive advantages include breathtaking purity, stringent impurity limits, and compliance with global standards. This first-rate API is trusted by major health institutions, leading medicine manufacturers, and innovative research organizations worldwide, who demand high efficacy for patient care. Choose Vildagliptin for grandiose reliability and performance in diabetic therapeutics.
Export Markets, Shipping, and Packaging Details for Vildagliptin API
Our Vildagliptin API is shipped to prime export markets including North America, Europe, Southeast Asia, and Africa, ensuring broad international reach. For your assurance, samples are available free of charge and processed promptly for evaluation. Each batch is securely packaged in HDPE drums with double polyethylene bags (1/5/10 kg or as required), ensuring safe transportation and meeting strict regulatory demands. Trust our service to deliver competitive, globally compliant products to you.
Vildagliptin API: Powerful Antidiabetic Excellence
Vildagliptin API is used for the effective management of type 2 diabetes mellitus, delivering pronounced glycemic control in pharmaceutical formulations. Its competitive advantages include breathtaking purity, stringent impurity limits, and compliance with global standards. This first-rate API is trusted by major health institutions, leading medicine manufacturers, and innovative research organizations worldwide, who demand high efficacy for patient care. Choose Vildagliptin for grandiose reliability and performance in diabetic therapeutics.
Export Markets, Shipping, and Packaging Details for Vildagliptin API
Our Vildagliptin API is shipped to prime export markets including North America, Europe, Southeast Asia, and Africa, ensuring broad international reach. For your assurance, samples are available free of charge and processed promptly for evaluation. Each batch is securely packaged in HDPE drums with double polyethylene bags (1/5/10 kg or as required), ensuring safe transportation and meeting strict regulatory demands. Trust our service to deliver competitive, globally compliant products to you.
FAQs of Vildagliptin API:
Q: How is the identity and quality of Vildagliptin API verified?
A: The identity of Vildagliptin API is verified using advanced HPLC and IR spectroscopy methods, while its purity, impurity levels, and other parameters are confirmed via Certificate of Analysis provided for each batch.Q: What competitive advantages does your Vildagliptin API offer compared to others?
A: Vildagliptin API provides breathtaking purity (99%), minimal impurities (0.2%), and is manufactured in a GMP-compliant facility, ensuring reliable, first-rate quality for pharmaceutical applications.Q: Where is your Vildagliptin API exported and how is it shipped?
A: Our Vildagliptin API is exported globally, including to North America, Europe, and Asia, and is shipped in secure HDPE drums with double polyethylene bags to ensure safe transportation and storage.Q: What is the process for obtaining a sample of Vildagliptin API?
A: You may request a sample free of charge; simply contact us with your details and our team will arrange prompt processing and shipping for evaluation.Q: When should Vildagliptin API be stored and how long can it be used?
A: Vildagliptin API should be stored in a cool, dry place, protected from light. Its shelf life is 2 years from the date of manufacture when stored correctly.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Active Pharmaceutical Ingredients' category
We accept only bulk quantity orders with more than 25 Kgs.